Immunotherapy Products
Our solution in Molecular Inmunotherapy
According to the World Allergy Organization (WAO), the prevalence of respiratory allergies across different countries ranges from 10% to 40% of the population.
The World Health Organization (WHO) recognizes immunotherapy as the only effective treatment that directly targets the root cause of allergic diseases by inducing both clinical and immunological tolerance to allergens.
The efficacy of allergen immunotherapy treatments is long-term. The recommended vaccination period ranges from 3 to 5 years, although the improvement of symptoms can be observed from 5 months. The specialist is responsible for annually assessing the condition of each patient in order to decide on the duration of the vaccination period in each case.
At DIATER, we are specialized in advanced treatments designed to provide long-term relief and address the underlying causes of allergic conditions.
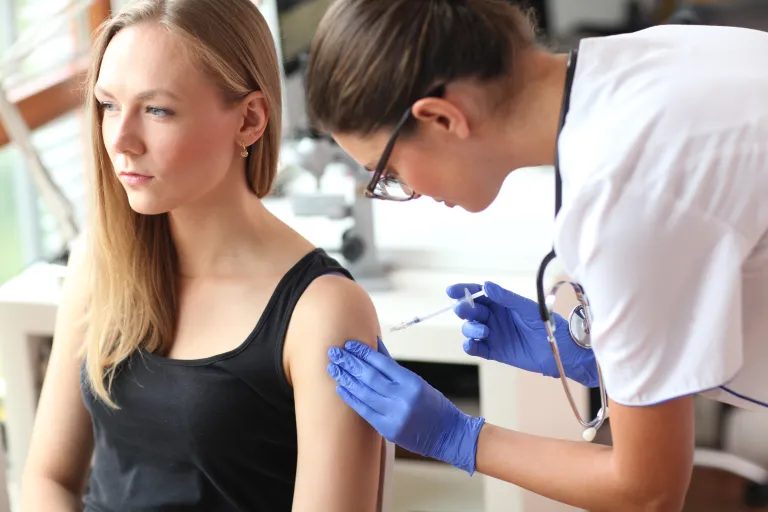
Subcutaneous Immunotherapy
This treatment involves monthly subcutaneous injections of the allergen/s to which the patient is sensitized. It requires administration by specialized healthcare professionals.
Molecular Immunotherapy
DIATER laboratories is pioneer in the use of native purified proteins (allergens) for molecular immunotherapy. This therapy offers a highly precise approach to allergy treatment by targeting the underlying cause of allergic reactions. With its advanced formulation, it provides a more personalized and effective solution for managing allergies.
Molecular Immunotherapy: A cutting-edge subcutaneous treatment.
Adverse Reactions to Subcutaneous Immunotherapy (SCIT)
Local Reactions
Local reactions are the most common and occur at the injection site. They include erythema, edema, warmth, inflammation, and pruritus. These reactions can be classified as:
- Immediate Reactions: Occurring within 30 minutes post-injection. They are considered significant if the reaction diameter exceeds 5 cm in adults and 3 cm in children.
- Delayed Reactions: Appearing after 30 minutes post-injection. They are deemed relevant when the induration diameter is greater than 10 cm in adults and 7 cm in children.
- Subcutaneous Nodules: Typically associated with vaccines adsorbed onto aluminum hydroxide. These nodules usually resolve over time, though some organized nodules may persist. Their presence does not mean discontinuation of immunotherapy.
Systemic Reactions
Systemic reactions involve symptoms affecting organs distant from the injection site. Symptoms can range from sneezing or isolated urticarial lesions to anaphylactic shock. They may occur immediately (within 30 minutes) or be delayed (hours or even days) after administration.
The World Allergy Organization (WAO) has established a grading system for systemic reactions due to subcutaneous immunotherapy, which is endorsed by current European immunotherapy guidelines. This classification assists in standardizing the assessment and management of such reactions.
Intradermal Immunotherapy
Intradermal Immunotherapy: marks the beginning of a new era in allergy care

Developed and patented by DIATER Laboratories, this innovative treatment requires fewer doses compared to conventional immunotherapy protocols. It works by directly targeting the immune system without the need of adjuvants, offering a faster and more efficient way to manage respiratory allergies.
Sublingual Immunotherapy
This treatment involves administering the allergen/s through the sublingual mucosa (under the tongue). It is a patient-managed regimen that is self-administered daily at home.
Adverse Reactions to Subcutaneous Immunotherapy (SCIT)
Sublingual immunotherapy is generally well tolerated by most patients; however, some individuals may experience adverse reactions.
Local Reactions
These are common but typically mild, occurring at or near the site of administration. They can manifest immediately or be delayed. Such reactions often appear during the initial doses and tend to diminish with continued treatment.
Reported local reactions include:
- Oral Mucosal Discomfort: Itching or swelling in the oral or pharyngeal mucosa.
- Gastrointestinal Discomfort: Nausea, vomiting, diarrhea, abdominal pain.

Systemic Reactions
These are rare and occur at sites distant from the administration area. Systemic reactions can be immediate or delayed.
Overall, SLIT is considered a safe and effective treatment for allergic rhinitis and asthma, with a favorable safety profile compared to subcutaneous immunotherapy (SCIT).
References
1 Alvaro-Lozano, Montserrat et al. “EAACI Allergen Immunotherapy User's Guide.” Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology vol. 31 Suppl 25,Suppl 25 (2020).
2 Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010.
3 Calderón, M A et al. “European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real-life clinical assessment.” Allergy vol. 72,3 (2017).